Quick Summary: Are you a developer, product manager, or business leader in the HealthTech sector? Are you looking to bring your digital health app to the UK healthcare market? Is your team struggling to understand or comply with the complex and evolving UK regulatory landscape, such as the NHS and MHRA app compliance checklist? You’re not alone, and this guide on building compliant HealthTech apps in the UK is designed to help you through the entire process.
With great opportunity comes greater responsibility. This is why, when our team at CMARX builds a digital health application for the UK market, we ensure healthcare app compliance UK with proper standards, such as those outlined in the Medicines and Healthcare products Regulatory Agency (MHRA medical device app regulations) and the NHS digital guidelines for app developers. It is a foundational practice needed for ensuring patient safety, data security, adoption by NHS organizations, and long-term viability. At CMARIX, our mobile app development services are designed and developed to meet these regulatory standards from the outset.
In this blog on building compliant healthcare apps UK, CMARIX walks you through a comprehensive roadmap, covering the regulatory environment, standards to meet, practical compliance strategies, certification processes, common challenges (and how to overcome them), and a future-facing view of what’s changing. If you are a developer, product manager, or business leader in the HealthTech sector looking to operate in the UK and understand the market beyond healthcare technology trends, this guide is relevant to you.
Why Compliance Matters (Beyond “Just doing what the law says”)
- Patient trust: Users expect digital health tools to be safe, reliable, and respectful of their data; compliance signals that. As of December 2023, the NHS App had 33.6 million registered users in England, which represents approximately three-quarters of the adult population. Monthly log‑ins rose from 14.8 million in December 2022 to 25.8 million in November 2023.
- NHS adoption: Trust and procurement by NHS organisations often hinge on meeting baseline digital health standards such as those set by the NHS.
- Market access: Without the right regulatory clearances (for example, the UKCA mark), we may not be able to market or sell in Great Britain.
- Competitive edge: Compliance becomes a differentiator: being “NHS-ready” or “regulator-compliant” is a selling point.
Fundamentals to Develop Compliant Healthtech Apps in UK
1. UK Medical Device Regulations (UK MDR 2002)
The core legislation for medical device software regulations UK, or Great Britain (England, Wales, Scotland), is the Medical Devices Regulations 2002. Under this law, software that qualifies as a “medical device” must comply with specific obligations.
For instance, any software used for diagnosing, monitoring, or treating a health condition must comply with certain obligations. It is ideal to consult with an expert healthcare software development company with experience working with UK compliance on how to take this forward.
Key obligations include:
- Classifying your device (Class I, IIa, IIb, III) based on risk
- Conducting conformity assessments
- Holding a Technical File, performing clinical evaluation, risk management (often aligned to ISO 14971)
- Establishing a Quality Management System (e.g., aligned to ISO 13485)
- Implementing post-market surveillance, vigilance, and incident-reporting
2. MHRA Guidance & UKCA Marking
The MHRA regulates medical devices and software in the UK. Software that meets the definition of a “medical device” or software as a medical device (SaMD) must often obtain the UK Conformity Assessed (UKCA) mark (or operate under interim arrangements) in Great Britain.
The official guidance “Software and artificial intelligence (AI) as a medical device” from the MHRA explains how standalone software and mobile apps may qualify as medical devices.
The classification of software-based medical devices (SaMD) is being aligned with the framework established by the International Medical Device Regulators Forum (IMDRF).
Important practical note: If your app has an intended medical purpose (diagnosis, monitoring, treatment, etc.), you must carefully determine whether you are within the scope of the medical-device regulations (and hence UKCA marking) or outside that scope (non-medical wellness, lifestyle, etc.). The intended use defines the regulatory classification. Get in touch with a medical software development company like CMARIX to gain more valuable insights, tips, and important updates.
3. NHS Digital Standards & DTAC
The NHS (via its digital transformation teams) has established minimum baseline criteria for digital health technologies that will be procured by NHS organisations. One such framework is the Digital Technology Assessment Criteria (DTAC). The DTAC covers five major domains: clinical safety, data protection, technical security/cybersecurity, interoperability, and usability & accessibility. Developers can use DTAC as a self-assessment or prepare evidence ahead of procurement by NHS organisations.
4. GDPR & Health App Data Privacy UK
| Requirement | What it Means |
| Get permission | Only use data when there’s a legal reason, and ask for clear consent if needed. |
| Be transparent | Explain to users how their data is used and what rights they have. |
| Keep it secure | Protect data with encryption and control who can access it. |
| Privacy first | Build systems that protect privacy from the start. |
| Check risks | Do a Data Protection Impact Assessment (DPIA) when handling high-risk data. |
| Plan for issues | Have a clear plan to address data breaches quickly and effectively. |
Strategic Approach to Comply with UK HealthTech App Development Standards
Compliance doesn’t come after you build the app; it needs to be woven into your development lifecycle from the start. Below is a logical approach.
Early Planning & Risk Management
- Define intended purpose early: What exactly will your app do? Will it diagnose, monitor, and treat? This determines whether you are subject to medical-device regulation. CMARIX clients can also hire mobile app developers who specialize in building compliant healthcare apps in the UK.
- Perform a risk assessment: Identify clinical risks (e.g., misdiagnosis, wrong therapy), data risks (data loss, hack), and technical risks (software bug, platform failure). Use ISO 14971 as a model.
- Data Protection Impact Assessment (DPIA): For an app handling health data, perform a DPIA to identify/mitigate data-protection risks.
- Governance structure: Assign roles for regulatory compliance, clinical safety officer, data protection officer (if applicable), and manage post-market surveillance.
Building a Compliance Framework
- Document governance: Create Standard Operating Procedures (SOPs) for device version control, incident reporting, change-control, and cybersecurity patching.
- Quality Management System (QMS): If subject to device regulation, you’ll need a QMS aligned with ISO 13485.
- Maintain a Technical File: For medical devices, you must maintain a technical file that includes software architecture, verification & validation records, clinical evidence, a risk-management file, user requirements, and installation/maintenance instructions.
- Versioning & change control: Software updates are frequent; however, under medical-device regulations, changes may require reassessment of risk and, in some cases, regulatory notification.
Clinical Evaluation and Evidence Generation
Your app may require clinical evidence to demonstrate its safety and effectiveness. Key considerations:
- Clinical studies: For higher-risk devices, randomized controlled trials or structured observational studies may be required.
- Real-world evidence (RWE): For digital health apps, it is increasingly acceptable to collect RWE (pilot data, user feedback, real-world usage metrics) to supplement formal clinical data.
- Transparency for AI/ML: If your app uses ML or AI, the MHRA’s guidance focuses on transparency, explainability, “good machine-learning practices,” and predetermined change-control plans (PCCPs).
- Documentation: Store clinical-evaluation reports, summaries of clinical data, and any adverse events; these form part of your submission to the MHRA and are important for post-market tracking and monitoring.
Technical Development and Security
- Secure coding practices: Utilize threat modeling, code reviews, and penetration testing. Infrastructure should be hardened.
- Encryption & authentication: Utilize encryption at rest and in transit, employ strong user authentication, and implement effective session management.
- Interoperability: Digital health apps often need to integrate with NHS systems, including Electronic Health Records (EHRs) and national services. Utilize interoperability standards, such as FHIR (Fast Healthcare Interoperability Resources) and HL7, in conjunction with other key APIs. You can also consider using the services of a professional healthcare CRM software development agency to achieve seamless integration with clinical and admin workflows.
- Cybersecurity standards: For NHS procurement, you will need to demonstrate compliance with frameworks such as the Data Security & Protection Toolkit (DSPT).
- Usability & Accessibility: Ensure your app meets accessibility requirements (e.g., WCAG) and usability for clinicians/patients.
- Testing & validation: Software verification and validation aligned with the software lifecycle standard, such as IEC 62304, where applicable.
Certification and Regulatory Approval Process
Conformity Assessment Procedures
- Determine classification – Use MHRA guidance to decide if your app is a medical device and what class (I, IIa, IIb, or III) it falls under, based on risk & intended use.
- Align with essential requirements – Ensure your app meets the “Essential Requirements” in UK MDR 2002 / Annex I (design, manufacturing, risk, usability).
- Choose conformity route – For Class I (non-sterile, non-measurement), you may self-declare; for higher classes, you’ll need a UK Approved Body (akin to a notified body) to assess your technical file, QMS, etc.
- Prepare technical documentation – Include software requirements specification, architecture, verification & validation, clinical evidence, risk management file, usability studies, and interoperability documentation.
- UKCA marking & registration – Once conformity is achieved, you affix the UKCA mark and register with the MHRA (for many devices).
- Affix labeling & instructions – Ensure your app has appropriate labeling, intended-use statement, user instructions, traceability, and version control.
UKCA Marking & MHRA Registration
Ensure you understand whether your app qualifies as a medical device or not. If it does, you normally must follow the above pathway. The UKCA mark is needed in Great Britain for many devices. However, current transition arrangements may allow some CE-marked devices to continue, but you should check with the current MHRA timelines first.
You will also likely need to register with the MHRA device database (for manufacturers placing devices in the UK market) and submit a report that covers measures for field-safety corrective actions and incidents.
NHS Digital Adoption and DTAC
| DTAC Area | Summary |
| Clinical Safety | Identify and manage risks to keep patients safe and monitor them continuously. |
| Data Protection | Follow GDPR: use encryption, do DPIAs for high risks, and let users control their data. |
| Technical Checks | Maintain strong cybersecurity, manage software updates carefully, secure APIs. |
| Interoperability | Use FHIR or HL7 standards for smooth NHS system integration. |
| Usability & Accessibility | Design apps that are easy to use and meet accessibility standards (WCAG). |
| DTAC Benefit | Meeting DTAC helps prove compliance to NHS buyers and speeds up approval and procurement. |
Our Solutions Against Challenges To Develop Compliant Healthtech Apps in UK
Navigating the Complex Regulatory Environment
Challenge: Guidance for digital health, particularly in the areas of software and AI, evolves constantly, making it easy to misclassify an app or overlook a requirement.
CMARIX Solution:
CMARIX stays ahead of regulatory changes by closely monitoring MHRA updates and working with digital health compliance experts. We map all regulatory obligations early and maintain a compliance roadmap. Regulatory checkpoints are integrated into agile sprints, ensuring every major release is assessed for compliance needs.
Clinical Validation & Evidence Generation
Challenge: Clinical trials are costly and time-consuming, yet regulators and the NHS expect solid evidence.
CMARIX Solution:
CMARIX combines formal studies with real-world evidence, including pilot deployments and data analysis. We work with clinical research organisations or NHS trusts to conduct a few pilot studies. For AI/ML-based healthcare applications, our team ensures there is transparency, traceability, and explainability of model decisions.
Security and Privacy Considerations
Challenge: Health apps face intense scrutiny over data protection and cybersecurity.
CMARIX Solution:
CMARIX implements robust security measures, including encryption, role-based access controls (RBAC), and audit logs, to ensure the highest level of security. Regular penetration testing and vulnerability assessments are carried out, and frameworks such as DSPT are utilized for NHS integration. Staff and contractors are trained on security best practices and incident response.
NHS Interoperability Integration
Challenge: Connecting apps with NHS EHRs or digital infrastructure can be complex and resource-heavy.
CMARIX Solution:
CMARIX adopts interoperability standards, such as FHIR and HL7, from the outset and develops a structured API strategy. Our teams combine with NHS digital experts or intermediaries to ease the integration process. We also implement robust API governance, versioning, and change management to ensure seamless long-term operation.

Future Outlook & Emerging Technologies (2026 and Beyond)
AI and Machine Learning-Powered Apps
- The MHRA’s guidance emphasises transparency and predetermined change-control plans (PCCPs) for ML/AI-enabled medical devices.
- Expect more focus on fairness, bias mitigation, model traceability, and performance monitoring in the post-market phase.
- If your app uses large-language models or generative AI for medical purposes, note that the MHRA clarifies: LLMs adapted for medical purposes are likely to be regulated as medical devices.
- If you are an AI healthcare startup, ensure early alignment with MHRA guidance to avoid regulatory delays.
Post-Market Surveillance (PMS) & Real-World Evidence (RWE)
- Regulatory frameworks are shifting to require more dynamic post-market monitoring of software/AI devices, not just “once launched and done.”
- You’ll need systems to capture real-world performance, user-reported incidents, version change logs, user feedback, and periodic reviews.
- RWE can support claims of safety and effectiveness, but it is also a regulatory expectation.
Regulatory Convergence and International Alignment
- The UK is aligning certain software/AI device regulations with IMDRF frameworks.
- The current transition period allows some CE-marked devices to continue in GB until June 2030, but this may change.
- If we plan to market beyond the UK (EU, US), consider parallel regulatory strategies to maximise reach.
Emerging Technologies and Traceability
- Blockchain, ledger-based traceability, and automated audit trails are becoming increasingly relevant for health device version control and post-market traceability.
- With connected devices/internet-of-things (IoT), cybersecurity becomes even more important and may face upgraded regulatory scrutiny.
Step-by-Step Roadmap for Building Compliant Healthcare Apps in the UK
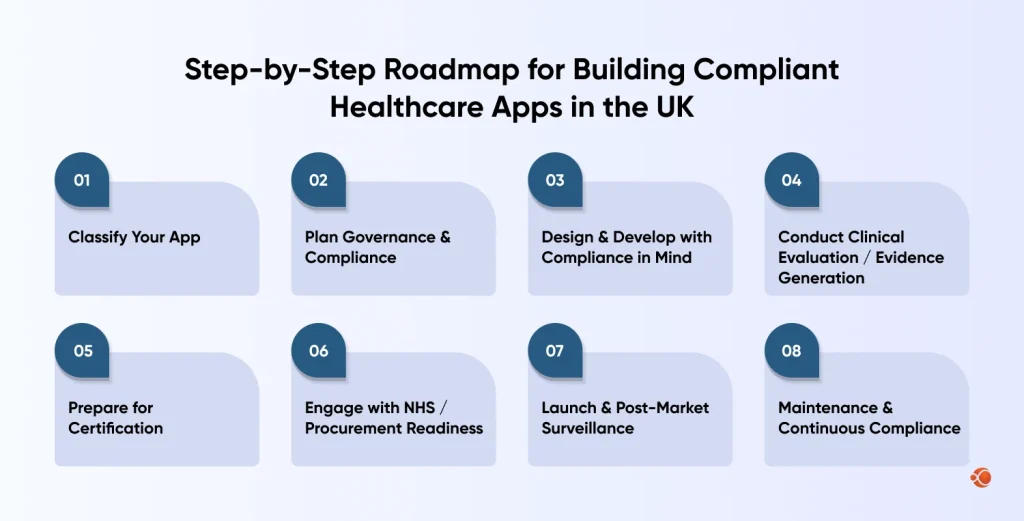
1. Classify Your App
- Define the actual purpose of building compliant healthcare apps in the UK: diagnosis, monitoring, or treatment?
- Determine if the software qualifies as a medical device under MHRA guidance.
- Assign risk class (I, IIa, IIb, III) based on potential harm.
2. Plan Governance & Compliance
- Establish roles: compliance lead, clinical safety lead, data protection officer.
- Set up a governance board or committee for oversight.
- Document SOPs: change control, incident reporting, version management, and cybersecurity patching.
3. Design & Develop with Compliance in Mind (“by-design”)
- Embed clinical safety, data protection (privacy by design), security, interoperability from day one.
- Select the appropriate standards: ISO 14971 for risk management, IEC 62304 for software lifecycle management, HL7/FHIR for interoperability, and WCAG for accessibility.
- Develop software verification & validation plans, usability tests, and security penetration tests.
4. Conduct Clinical Evaluation / Evidence Generation
- Draft a Clinical Evaluation Plan (CEP) aligned with device risk and complexity.
- Collect clinical data, pilot-study data, or real-world evidence (RWE).
- If AI/ML is used, document explainability, fairness, and performance monitoring.
5. Prepare for Certification
- Assemble the Technical File: software specs, architecture, V&V records, risk management, clinical evidence, usability evaluation, interoperability documentation.
- If you are in a higher risk class, select a UK-approved body and submit for conformity assessment.
- Prepare QMS (ISO 13485) if required.
- Decide on the UKCA mark and MHRA registration.
6. Engage with NHS / Procurement Readiness
- Conduct a DTAC self-assessment: Start by gathering data on clinical safety, data protection, technical security, interoperability, and usability.
- Prepare supporting documentation: This includes user manuals, training materials, integration guides, and security certificates.
- Plan for procurement: NHS local trusts will require evidence, references, and integration pathways to secure NHS-approved health app recognition.
7. Launch & Post-Market Surveillance
- Once launched, monitor usage, collect user feedback, track incidents, record software changes, and update risk management accordingly.
- Implement a vigilance system: report serious incidents to the MHRA if needed.
- Maintain version control and change logs, and update the technical file whenever significant changes occur.
8. Maintenance & Continuous Compliance
- Conduct periodic audits, risk reviews, and DPIAs updates.
- Stay up to date with regulatory updates, such as software/AI regulatory reform, which may require adaptation.
- Ensure maintenance of interoperability compatibility (for example, if API standards evolve), security patches, and user training.
- Renew evidence, re-assess DTAC when new versions of your app are released.
Final Thoughts
Building compliant HealthTech apps in the UK in 2026 is not about checking a few list items off a list of regulations and calling it a day. It is a conscious and deliberate effort to ensure a safe, secure, and interoperable design throughout the entire process.
When we align with the MHRA, NHS, and data protection standards from the outset of the project, compliance with regulations becomes much easier. As a side benefit, we also build trust, facilitate NHS procurement, and position ourselves for sustainable success.If you follow the roadmap in this guide, you will be well-positioned to bring a HealthTech solution that is not only innovative but also safe, trusted, and ready for adoption across the UK. Get in touch with our team to discuss your healthcare app ideas, and we will take it from there.
FAQs on Building Compliant Healthcare Apps in the UK
What regulations govern HealthTech apps in the UK?
HealthTech apps in the UK must follow medical device regulations, NHS digital standards, and data protection laws. This includes rules for clinical safety, data privacy under GDPR, and technical security to ensure apps are reliable and safe for patients.
What is NHS DTAC and why is it important?
NHS DTAC is a set of criteria used by the NHS to assess digital health tools. It ensures apps are clinically safe, protect patient data, work securely, are easy to use, and can connect with NHS systems. Meeting DTAC standards is important for NHS approval and trust.
What steps are involved in developing a compliant HealthTech app in the UK?
Developers should first understand the regulations and user needs, design with safety and privacy in mind, build a prototype, conduct clinical testing, implement robust security measures, and plan for ongoing monitoring and improvement to maintain compliance.
How can developers ensure interoperability with NHS systems?
Interoperability for building compliant healthcare apps UK requires developers to integrate NHS-approved data standards, such as FHIR, and secure APIs to facilitate seamless data exchange. Developers must adhere to NHS guidelines for data sharing, authentication, and system integration to facilitate seamless, secure data exchange.
How do I get MHRA approval for my medical app?
To get MHRA approval, classify your app’s risk under UK medical device rules, compile clinical and technical evidence, complete conformity assessments, submit documents to MHRA, and, after approval, apply the UKCA mark indicating regulatory compliance.







