Quick Summary: Generative AI in life sciences is transforming the way regulated software is smarter, built, faster, and with greater confidence. From automating documentation and validation to strengthening compliance and traceability, it helps businesses cut delays and deliver high-quality systems without compromising safety or regulatory standards.
Life sciences software development has always been difficult. Teams must balance innovation with strict regulations, high expectations, and long validation cycles around safety and accuracy. Be it developing clinical systems, regulatory tools, or laboratory platforms, there is less chances of error, and even less room for delays.
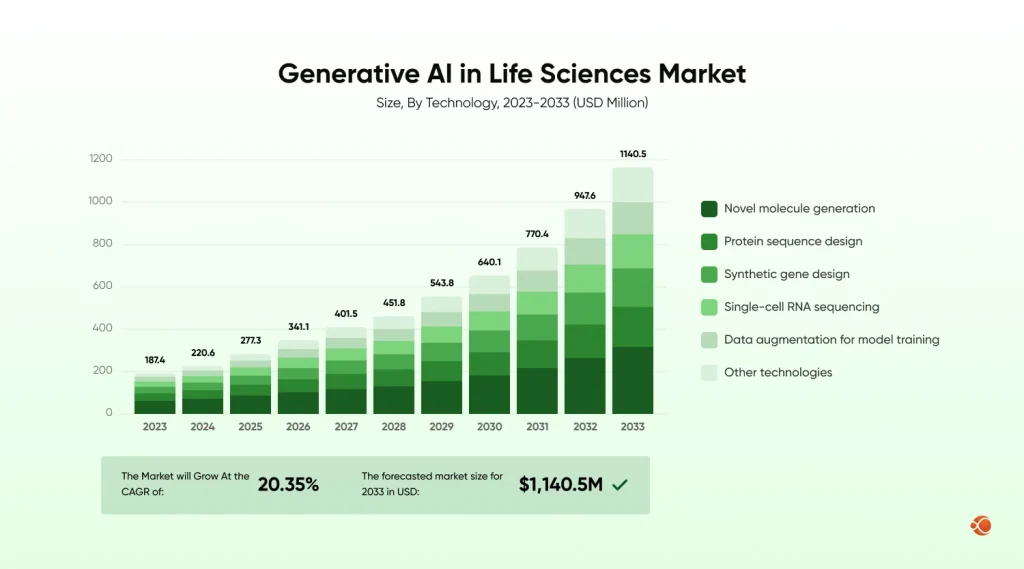
At the same time, organizations are under pressure to deliver faster, with reduced cost, and adapt to constant regulatory change. This is where Generative AI in life sciences is starting to make a true impact. The global life sciences market will grow to USD 1140.5 by 2033, up from USD 341.1 in 2026, with a CAGR of 20.35% over the forecast period.
Generative AI can create new content from requirements and documentation to code and test cases. It does not just follow rules; it learns patterns and produces meaningful outputs based on context. For life sciences software teams, this means better consistency, fewer manual tasks, and more time to focus on complex decision-making.
In this blog, we’ll explore how generative AI fits into the life sciences software development lifecycle (SDLC), where it brings the most value, what risks to manage, and how organizations can adopt it responsibly.
Understanding the Life Sciences Software Development Lifecycle (SDLC)
Software development in life sciences looks very different from general software development. While most industries focus on user experience and speed, life sciences must focus on safety, traceability, compliance, and validation.
What the Life Sciences SDLC Looks Like
The life sciences SDLC process usually follows a structured and documented approach. The process begins with intensive requirement gathering sessions and progresses to architecture design, development, testing, deployment, validation, and maintenance. Each phase of the process includes approvals, documentation, and traceability.
Unlike consumer software, changes cannot be made casually. Even a small update may require impact analysis, regulatory documentation, and re-validation. This is necessary to comply with standards such as FDA 21 (Food and Drug Administration Title 21), CFR Part 11 (Code of Federal Regulations Title 21, Part 11), GxP (Good Practice), ISO 13485, and other regional and global regulations.
While this approach protects patient safety and data integrity, it also increases development cost and time.
Why Generative AI Matters for Life Sciences Organizations
This is where AI in life sciences software development becomes especially valuable. Gen AI helps businesses handle the heavy documentation load, reduce manual errors with smooth validation workflows. It can analyze regulatory texts, support traceability and draft structured documents, all while maintaining accuracy and consistency.
Artificial intelligence is more of a support system that accelerates work and reduces friction between teams, rather than being a substitute for human judgment. For companies that operate in a regulated space, this paradigm shift is not only about efficiency but also about competitiveness and compliance.
What Is Generative AI? A Practical Overview for Software Teams
Before exploring use cases, it’s important to understand what generative AI actually means and how it applies to software development.
How Generative AI Works in Software Development
Generative AI systems are trained on large volumes of data such as documents, code repositories, and technical content. From this data, they learn patterns and relationships,
allowing them to generate new outputs that resemble human-created content.
In software development, this means AI can:
- Draft documentation and reports
- Write and refactor code
- Create test cases
- Summarize complex technical information
- Provide contextual suggestions
Instead of simply automating repetitive tasks, generative AI supports higher-level work such as analysis, planning, and quality improvement.
Types of Generative AI Models Used in the SDLC
| Model Type | Common Use in Life Sciences SDLC | Example Products |
| Large Language Models (LLMs) | Writing documentation, requirements, code, and test cases | OpenAI GPT-4, Claude, LLaMA |
| Code Generation Models | Assisting developers with syntax, logic, and debugging | GitHub Copilot, Tabnine, CodeWhisperer |
| Text-to-Document Models | Creating SOPs, validation reports, and compliance documents | Google Document AI, Azure Document Intelligence, Nanonets |
| Data Synthesis Models | Generating synthetic clinical or operational data | Synthea, Gretel AI, Mostly AI |
| Conversational AI Models | Supporting internal help desks and knowledge search | ChatGPT, Google Dialogflow, IBM Watson Assistant |
Together, these technologies strengthen the broader use of artificial intelligence in life sciences or AI for life sciences, particularly in areas where accuracy, consistency, and documentation quality are critical.
CMARIX connects you with pre-vetted AI experts to turn ideas into production-ready solutions.
Contact UsHow Generative AI Is Reshaping the Software Development Lifecycle
Generative AI is no longer limited to pilot projects or experimentation. It is actively reshaping how teams work across the SDLC.
Key Benefits of GenAI Across the SDLC
Organizations adopting GenAI in SDLC are seeing improvements in various areas.
- Shorter development cycles and faster release timelines
- Better documentation quality and consistency
- Expanded test coverage and earlier defect detection
- Reduced time spent on routine tasks
- More focus on high-impact work like design, validation, and optimization
In life sciences, where delays can affect product launches, regulatory submissions, and patient access, these improvements have real business impact.
Adoption Challenges and Industry Trends
Generative AI adoption is not without challenges, despite its value. Teams may hesitate to trust AI-generated outputs. There are concerns about hallucinations, accuracy, data privacy, and regulatory acceptance. Integration with legacy systems can also be difficult.That said, adoption is accelerating. According to McKinsey, more than 88% of organizations now use generative AI in at least one business function, including healthcare and life sciences.
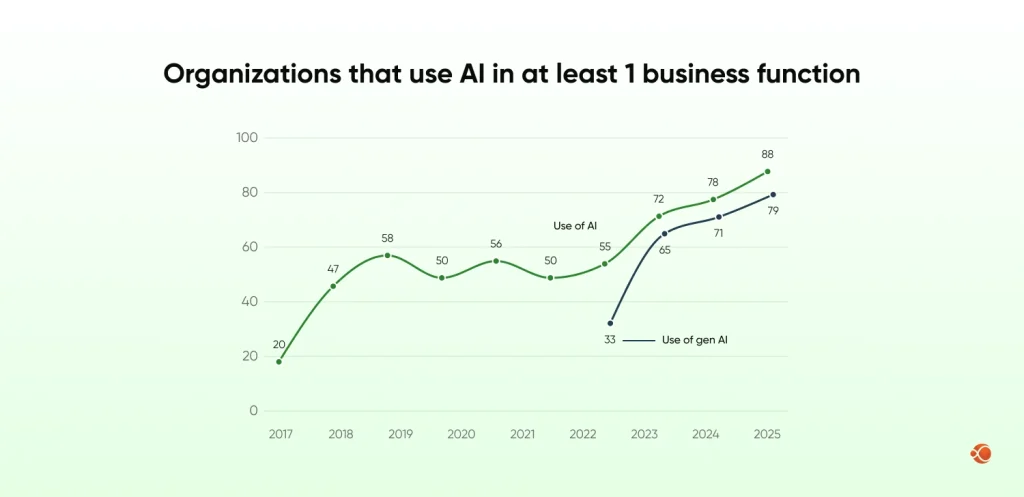
This shows that generative AI is moving from early testing into real-world use, even in regulated industries.
Phase-by-Phase Use of Generative AI in the Life Sciences SDLC
Now, let’s see how generative AI supports each phase of the life sciences SDLC in practice.
GenAI for Requirements Gathering and Analysis
Requirements are the foundation of any life sciences software project. They must be complete, clear, and aligned with regulatory expectations. Errors at this stage lead to rework, delays, and compliance risks later.
Gen AI supports this phase by analyzing stakeholder inputs, historical project data, and regulatory documents. It can draft user stories, functional, and non-functional requirements in structured formats. It can also highlight gaps, inconsistencies, or conflicting requirements.
Organizations begin their AI journey here, especially when working with a Generative AI solutions company, because better requirements lead to better outcomes across the entire lifecycle.
GenAI in Architecture and Design Planning
System architecture in life sciences must support scalability, security, performance and compliance. The design decisions affect not only development but also maintenance, validation, and long-term regulatory risk.
Gen AI can suggest architecture patterns based on project goals, regulatory needs, and technical constraints. It can draft design documents, support trade-off analysis and summarize design options. And also align design outputs with compliance and security standards.
With Generative AI integration services, organizations can integrate AI into their design workflows, helping teams move faster while maintaining consistency and quality.
AI-Powered Code and Feature Generation
Code generation is one of the most prominent uses of generative AI. AI-based tools can be used for code snippet generation, code refactoring, and coding standard enforcement.
In the life sciences domain, code generated by AI needs to be validated by human experts.
Nevertheless, it has been a massive success in terms of saving development time and ensuring consistency. Developers can now concentrate on system logic, risk, and performance.
Automated Testing, Validation, and Quality Assurance with GenAI
Testing and validation are the most resource-intensive phases of SDLC. Every change must be validated, every requirement must be tested, and every outcome must be documented.
Generative AI supports QA teams by generating test cases as per the requirements and code. It can draft validation protocols, identify coverage gaps, and suggest risk-based testing strategies. Also, help maintain traceability between requirements, tests, and results. This improves both speed and quality while maintaining regulatory rigor.
GenAI in Deployment and CI/CD Pipelines
Deployment is highly controlled in life sciences. Each release requires approvals, traceability and documentation. Unapproved changes or errors can lead to compliance issues. Generative AI can generate deployment documentation, monitor pipeline performance, analyze release risks, and suggest rollback strategies. Tools like:
- GitHub Copilot helps teams write and review deployment scripts faster
- Datadog and Dynatrace use AI-powered monitoring to detect anomalies, predict failures, and improve system reliability.
- For CI/CD orchestration, tools like GitHub Actions, Azure DevOps, and Jenkins can integrate AI-driven checks for quality, security, and compliance.
When combined with modern CI/CD practices, AI helps businesses maintain compliance while still benefiting from automation.This is especially valuable for organizations offering healthcare application development, where reliability and uptime are important.
AI-Driven Maintenance, Support, and Documentation Automation
Once the software is live, the maintenance works continue. Organizations must manage updates, support tickets, audits, regulatory inspections, and documentation changes.
Generative AI can draft change requests, analyze support tickets, update documentation automatically, and generate knowledge base content. Also, it can assist with impact analysis and audit preparation. These capabilities help organizations scale operations without increasing headcount, a key benefit for growing AI healthcare startups.
Generative AI Use Cases in Life Sciences
Regulatory Compliance Documentation and Intelligence
One of the most time-consuming aspects of life sciences software development is regulatory documentation. Development teams are required to generate SOPs, compliance reports, validation plans, and audit reports, sometimes with a tight deadline, making it one of the most important gen AI use cases in healthcare.
The use of Generative AI will enable the generation of these documents, summarization of regulatory guidelines, tracking of regulatory updates, and mapping of requirements to compliance standards.
Various organizations use generative AI in clinical healthcare solutions to strengthen their regulatory workflows while maintaining accuracy and audit readiness.
Validation Test Plan and Protocol Generation
Validation is an operational and legal requirement in life sciences. It makes sure that systems perform as intended and meet regulatory expectations.
Generative AI can generate validation test plans from requirements and draft PQ (Performance Qualification), IQ (Installation Qualification), and OQ (Operational Qualification) protocols. And suggest test cases aligned with regulatory standards, maintain traceability between requirements, tests, and validation results.
When combined with generative AI in data science applications, validation processes can benefit from data-driven risk analysis and predictive insights.
Data Standardization Using AI (CDISC, HL7, FHIR)
Life science organizations depend on standardized data formats to make sure interoperability, regulatory compliance, and data integrity. Some of the common standards include:
- CDISC (Clinical Data Interchange Standards Consortium)
- HL7 (Health Level Seven)
- FHIR (Fast Healthcare Interoperability Resources)
Gen AI can map data between validated structures and standards, generate documentation, and detect inconsistencies. This simplifies integration across systems and improves data quality.
Automated Traceability and Audit Reporting
Traceability is the main part of compliance. Businesses must be able to track every requirement through design, development, testing and release.
Generative AI can automatically link requirements to tests, code, and documentation. It can generate audit reports, find traceability gaps, and support internal and external audits.
Many organizations use generative AI statistics to measure improvements in documentation quality, validation efficiency and audit readiness.

Top Generative AI Tools for the Life Sciences SDLC
| SDLC Phase | Top GenAI Tools | Primary Use |
| Research & Ideation | AlphaFold, Elicit | Protein modeling, literature review |
| Requirements & Design | ChatGPT, Claude | Requirements, system design |
| Development | GitHub Copilot, CodeWhisperer | Code generation & review |
| Data & Modeling | NVIDIA Clara, Aiforia | Imaging, genomics, bio data analysis |
| Testing & Validation | Diffblue, Mabl | Test case generation |
| Documentation | Notion AI, Scribe | Technical and regulatory documentation |
| Compliance & Monitoring | Cortical.io, Dynatrace | Compliance checks, system monitoring |
The above tools support risk forecasting, task planning, knowledge management, and team communication. Organizations that hire AI developers depend on these tools to coordinate distributed teams effectively.
Risk, Compliance, and Ethical Considerations in Using GenAI
Gen AI introduces new opportunities, but also new risks, specifically in regulated industries.
Managing Bias, Hallucinations, and Output Accuracy
There may be instances where the AI system produces incorrect or misleading information. In the field of life sciences, this has to be managed. It is important that organizations ensure human review of critical outputs, version control, and strong documentation of AI-generated content against trusted sources.
It is important that businesses ensure human review of critical outputs, validate AI-generated content against trusted sources, and maintain strong documentation and version control. AI systems should support human judgment, not replace it.
Intellectual Property Protection and Documentation Integrity
The use of generative AI also raises questions regarding the ownership of content generated through AI, the confidentiality of the data used to train AI, and the protection of proprietary information. Organizations need to develop policies and frameworks regarding access to intellectual property.
Validating AI Systems in Regulated Life Sciences Environments
AI tools that affect regulated processes may themselves require validation. This includes testing outputs against expected results, documenting system behavior, maintaining audit trails, and monitoring performance.
These steps help in ensuring that AI use aligns with regulatory expectations and quality standards.
Challenges of Implementing Generative AI in the Life Sciences SDLC
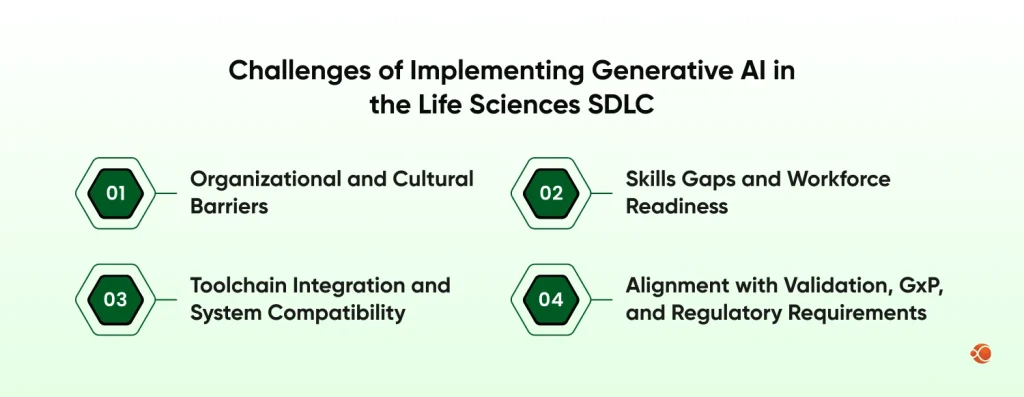
1. Organizational and Cultural Barriers
Common challenges include a lack of trust in AI outputs, concerns about outputs, resistance to change, and uncertainty about regulatory acceptance. Clear communication, strong leadership, and practical training help address these concerns.
2. Skills Gaps and Workforce Readiness
Various organizations lack experience with AI in regulated workflows or knowledge of risk management, AI, and data science expertise. Addressing these gaps requires hiring, training, and partnerships.
3. Toolchain Integration and System Compatibility
Life sciences environments often include complex legacy systems. AI tools integration into these environments can be difficult due to limited APIs, security constraints, data silos and performance concerns. Phased implementation and careful planning are important.
4. Alignment with Validation, GxP, and Regulatory Requirements
AI adoption must align with GxP(Good Practice) standards, validation frameworks and data integrity rules and audit readiness expectations. This requires close collaboration between quality, IT and regulatory teams.
Best Practices for Adopting Generative AI in Life Sciences Software Development
Starting with Proof of Concept and Pilot Projects
Instead of rolling out AI across the business all at once, begin with small, low-risk use cases. Make clear success metrics, document outcomes and operate in controlled environments. This builds confidence and experience before scaling.
Building Effective Human–AI Collaboration Models
Gen AI works best when it is paired with human expertise. Businesses should define clear roles for both humans and AI, make sure human review of critical outputs, train teams on effective AI use and encourage feedback and learning.
Continuous Monitoring, Evaluation, and Improvement
AI systems must be monitored to make sure compliance, accuracy, stability and continuous relevance. This requires governance frameworks, regular reviews and performance metrics.
Organizations working in artificial intelligence in the life sciences increasingly treat generative AI in life science market as a long-term capability rather than a one-time implementation.
Partner with CMARIX’s experienced AI developers to accelerate your roadmap.
Contact UsFuture of AI in Life Sciences Software Development
Generative AI is continuously evolving, and its role in life science software development is also expanding.
The Rise of Agent-Based and Autonomous SDLC
The future will see the use of AI agents that will manage activities in the SDLC, track risks and performance, initiate workflows and approvals, and provide recommendations for process optimization.
Creating an AI-Native Software Development Lifecycle
Organizations will adopt SDLCs that are AI-centric, with features such as continuous validation, AI-assisted analysis of requirements, and intelligent management of compliance and documentation
Real-Time Regulatory Intelligence and Compliance Automation
The generative AI will allow real-time tracking of regulatory updates, automated impact assessments, dynamic updates of policies, and proactive management of compliance.
AI-Driven Continuous Validation and Quality Monitoring
Validation will be continuous, rather than periodic. AI will monitor systems in real time, identify anomalies, initiate corrective steps, and ensure audit readiness.
Fully Autonomous Development Processes in the Life Sciences
In the long term, certain processes may be fully autonomous, with AI performing routine tasks and humans focusing on strategy and monitoring. This illustrates the overall expansion of generative AI in the life sciences market.
Why Choose CMARIX for Generative AI Software Development in Life Sciences
For implementing generative AI in regulated environments, it becomes important to choose the right development partner. CMARIX brings deep experience in life sciences and healthcare software development services, a strong understanding of validation and regulatory requirements, and proven expertise in AI and advanced technologies. Our approach focuses on safety, compliance, and long-term value instead of short-term organizations with effective, responsible, and compliant AI integration.
Conclusion
Gen AI in life sciences is already changing how software is built, tested, validated, and maintained. When adopted thoughtfully, generative AI helps businesses move faster, improve quality, reduce operational burden, and strengthen compliance, all without compromising safety or trust.
The key here is not just replacing human expertise, but also supporting it. With ideal strategy, governance, and tools, generative AI becomes a powerful ally in delivering smarter, safer, and more reliable life sciences software.
Abbreviations
- GenAI- Generative AI
- LSSD- Life Sciences Software Development
- SDLC-Software Development Lifecycle
- GxP- Good Practice
- CI/CD- Continuous Integration / Continuous Deployment
- CDISC- Clinical Data Interchange Standards Consortium
- HL7- Health Level Seven
- FHIR- Fast Healthcare Interoperability Resources
- CFR Part 11 — Code of Federal Regulations Title 21, Part 11
- FDA 21 — Food and Drug Administration Title 21
- PQ — Performance Qualification
- IQ — Installation Qualification
- OQ — Operational Qualification
FAQs on Generative AI in Life Sciences
What is Generative AI in life sciences?
Generative AI in life sciences means AI systems that make content, such as code and documentation. Test cases to support research, development, compliance, and healthcare software workflows. It is a key part of artificial intelligence for life sciences. It helps reduce manual effort while improving speed, consistency, and accuracy.
How is generative AI used in life sciences software development?
It is used to automate documentation, generate code and test cases, support validation, improve traceability, and assist with regulatory compliance. Many teams also use AI-powered search for life sciences to quickly access regulatory documents, research literature, and validation records.
How is GenAI used across the life sciences SDLC?
Gen AI supports requirements gathering, design, development, testing, deployment, maintenance, documentation, and audit reporting. It creates continuity and efficiency across every stage of the lifecycle.
Can GenAI help with regulatory compliance in life sciences?
Yes, it can draft compliance documents, support traceability, monitor regulatory compliance, and automate validation artifacts. This helps businesses stay audit-ready and reduce compliance risks.
Is generative AI secure for healthcare software development?
When implemented with proper validation, governance, and controls, generative AI can be used securely in regulated healthcare environments. Security depends on strong data protection, access controls, and model validation practices.